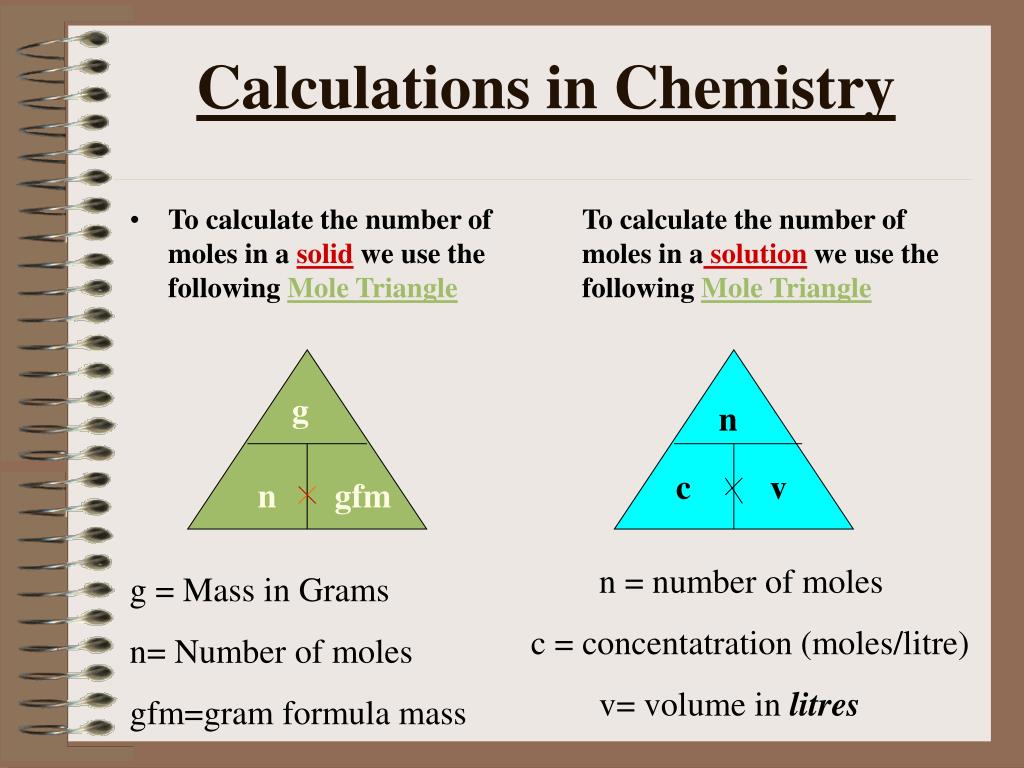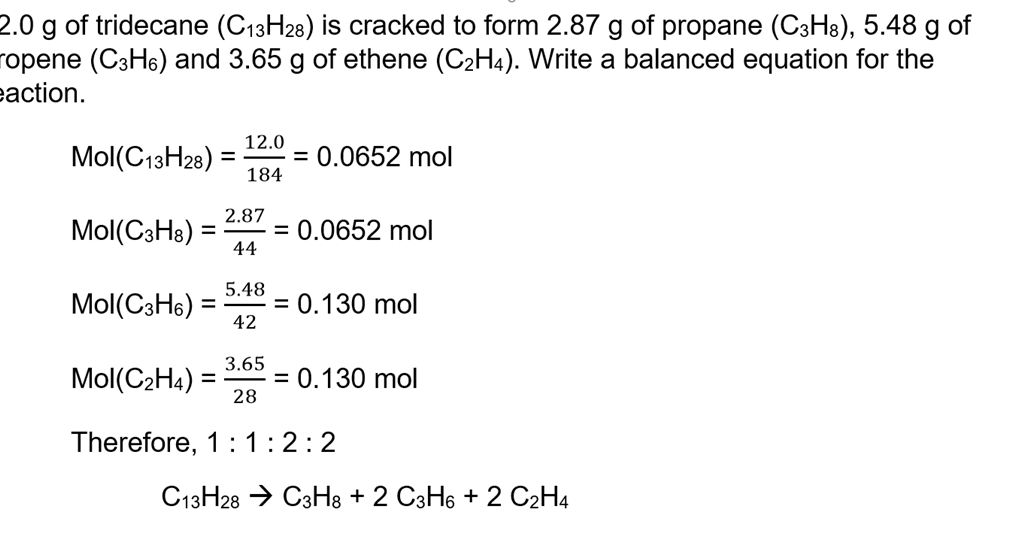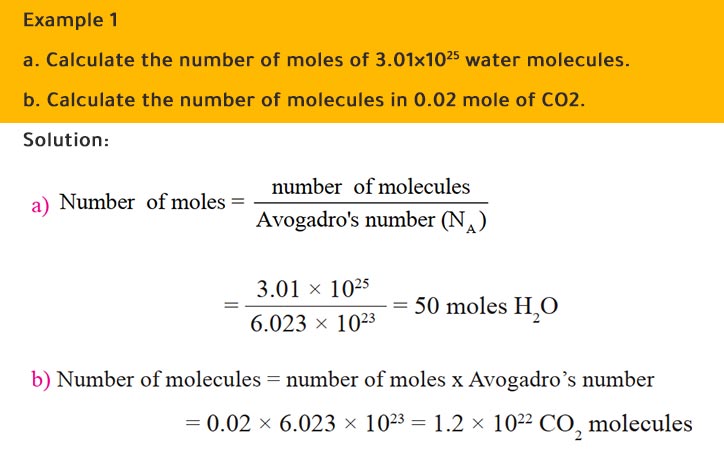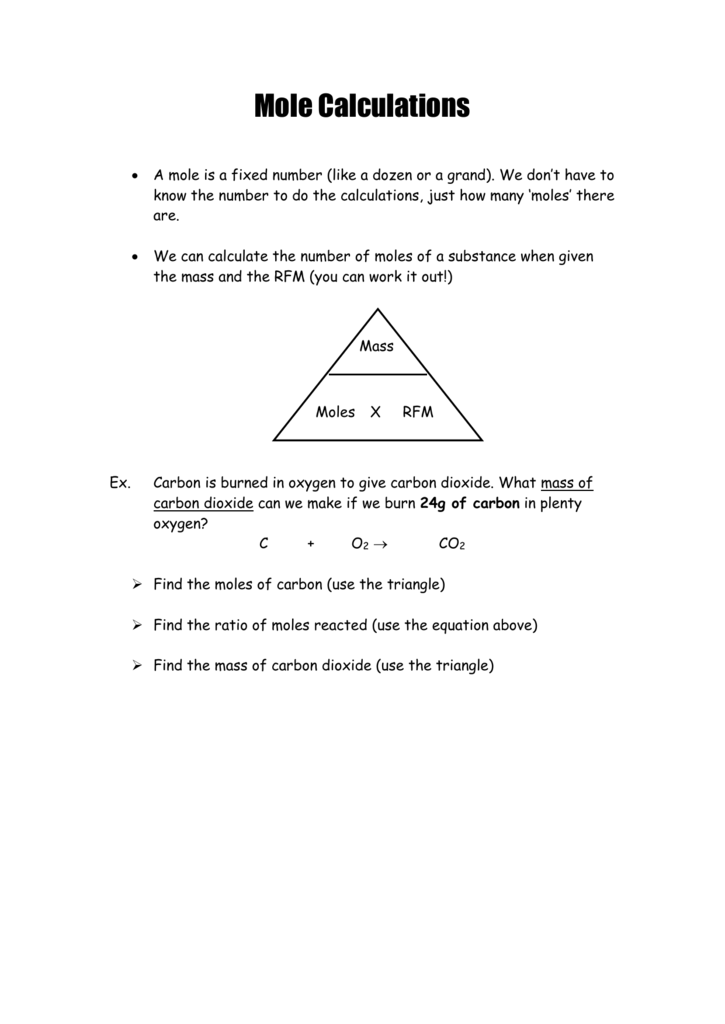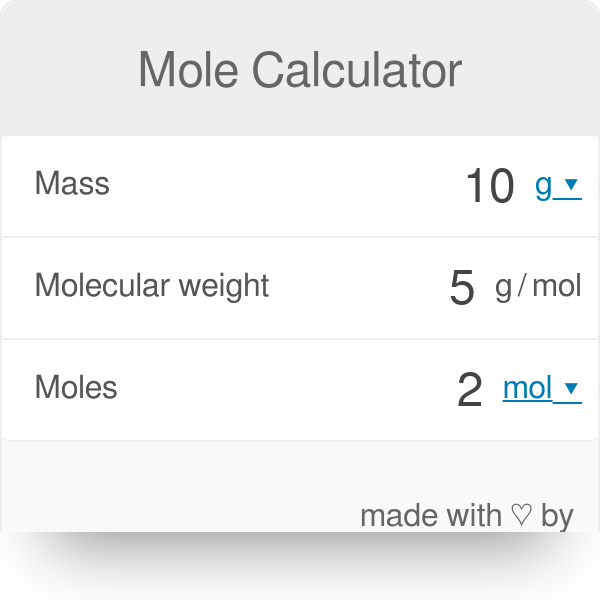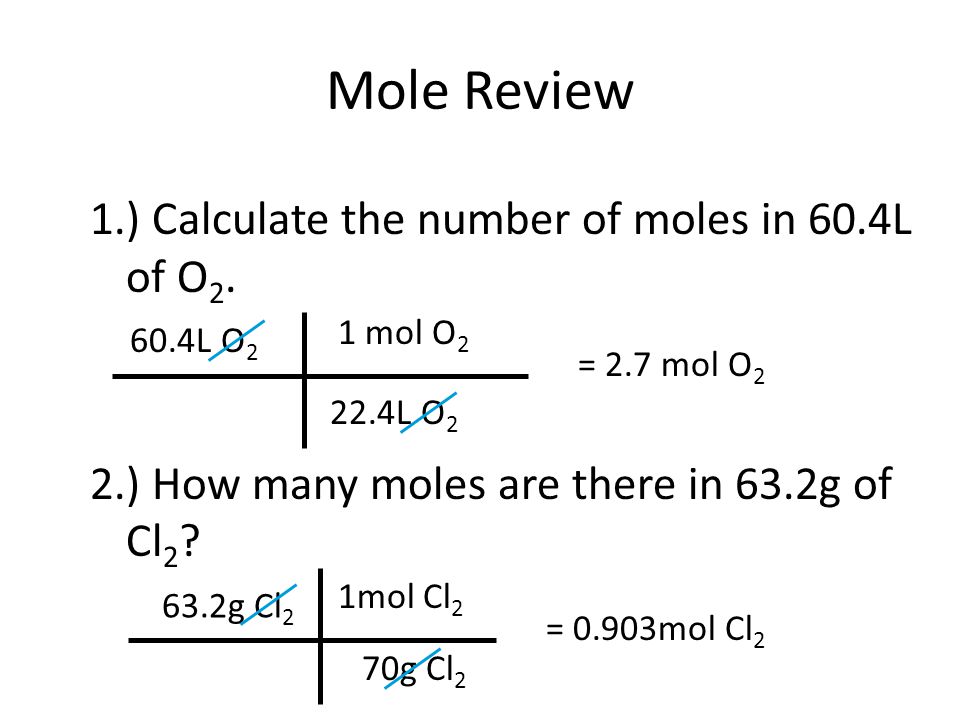
Mole Review 1.) Calculate the number of moles in 60.4L of O2. 2.) How many moles are there in 63.2g of Cl2? 1 mol O2 60.4L O2 = 2.7 mol O2 22.4L

How To Solve Most Mole Calculation Questions – Part 1 | O Level Chemistry Tuition | Leading Chemistry Tuition Centre

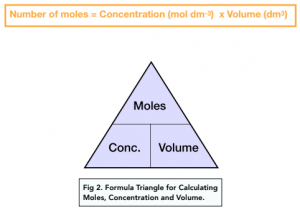
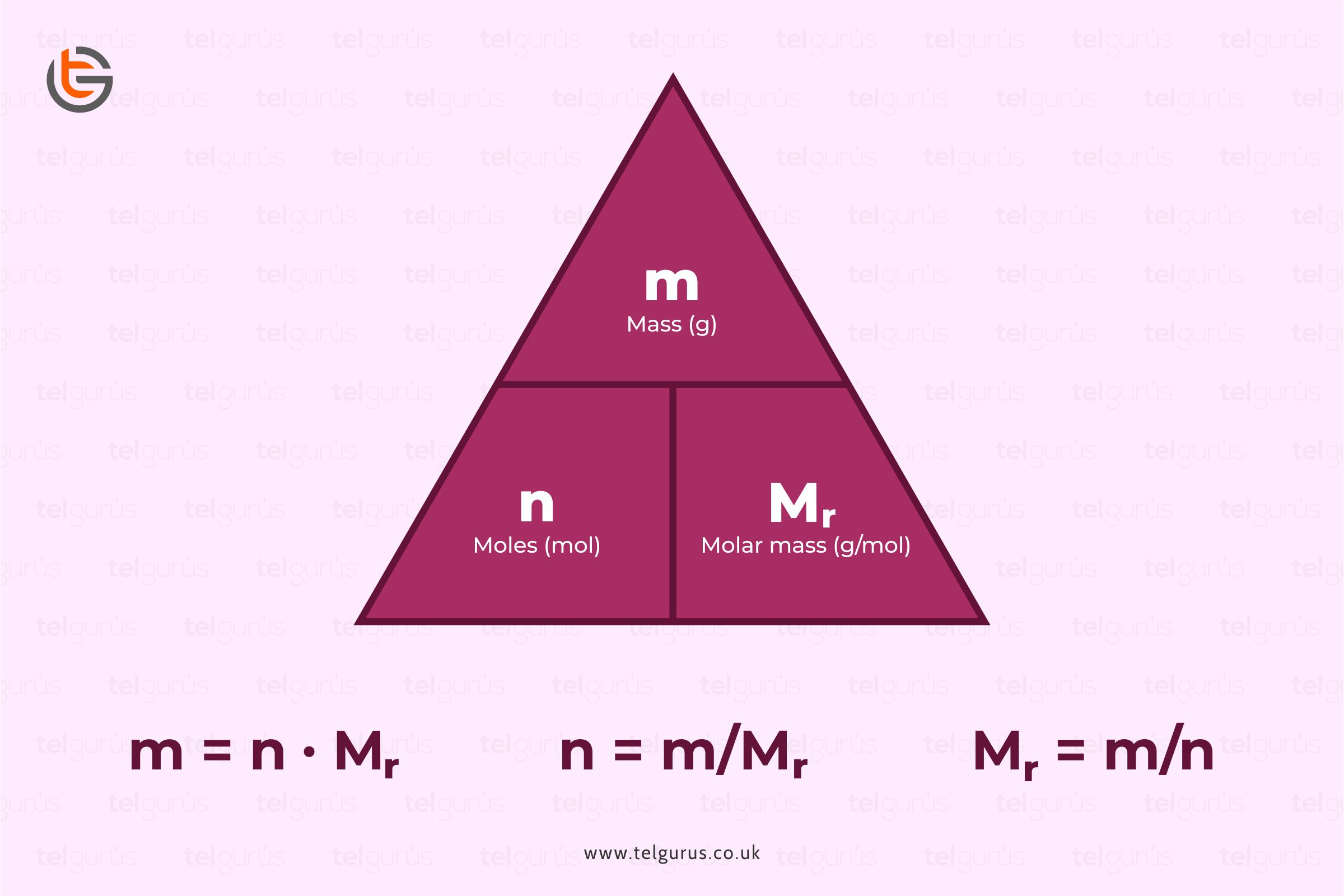
molar gas volume Avogadro's Law moles and mass calculations gcse chemistry calculations igcse KS4 science A level GCE AS A2 O Level practice questions exercises

Calculate the number of moles for the following: 52 g of He (finding mole from mass) 12.044 × 10^ 23 number of He atoms (finding mole from number of particles)