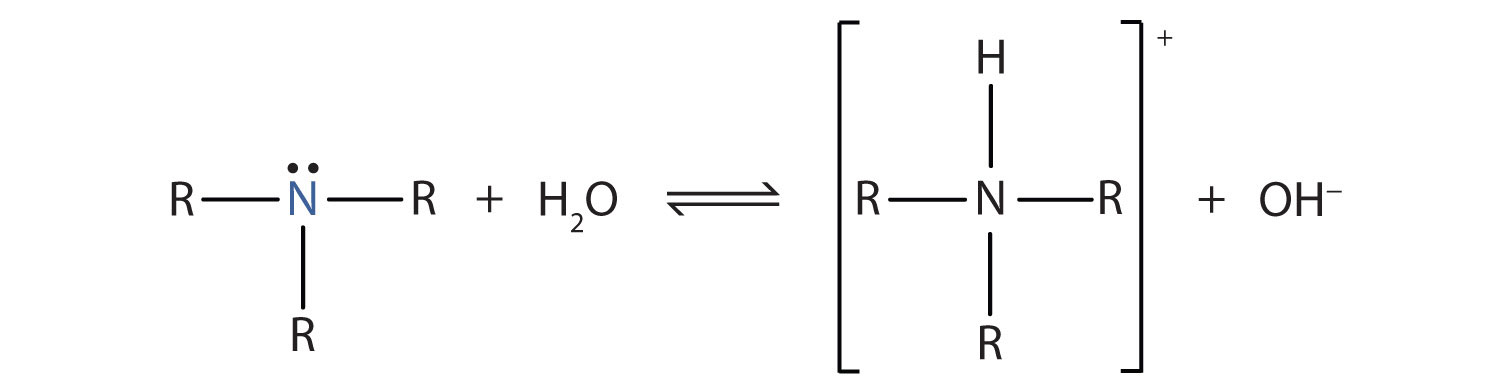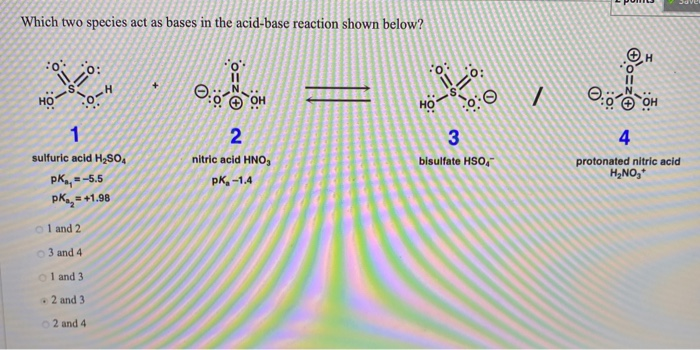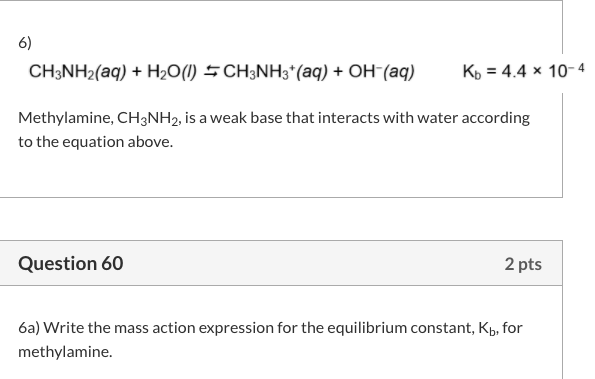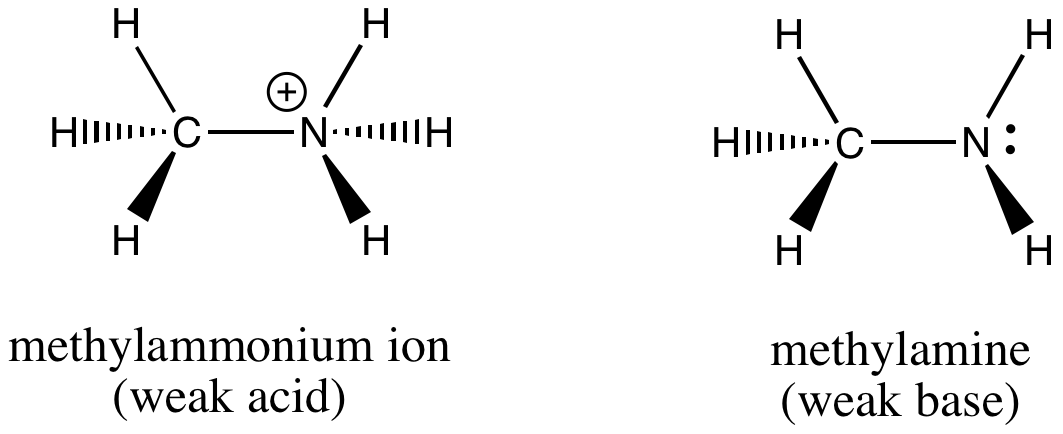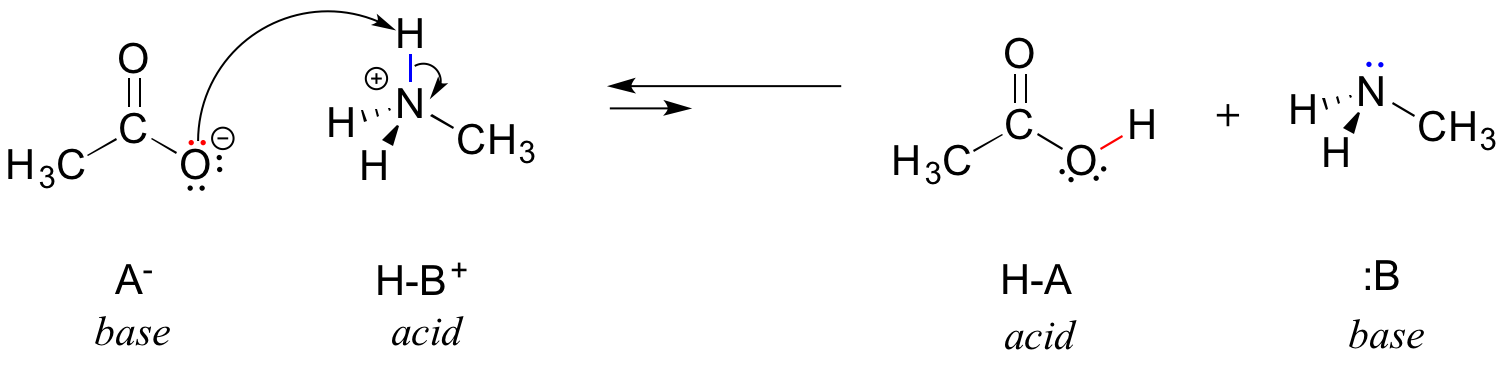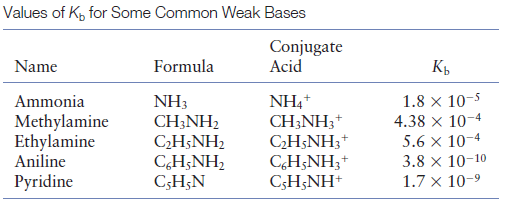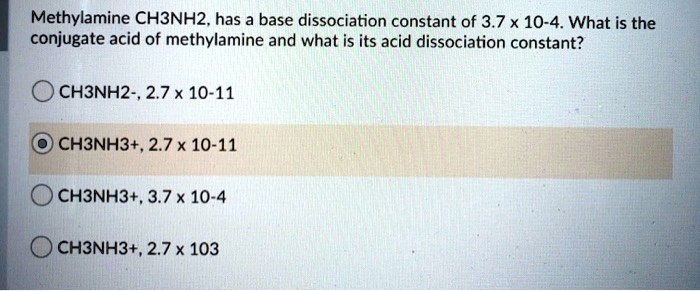
SOLVED: Methylamine CH3NH2, has a base dissociation constant of 3.7 x 10-4. What is the conjugate acid of methylamine and what is its acid dissociation constant? CH3NHZ -, 2.7 x 10-11 CH3NH3+,2.7
![SOLVED:In a 0.0100 M aqueous solution of methylamine, CH3 NH2, the equilibrium concentrations of the species are [CH3 NH2]=0.0080 mol / L and [CH3 NH3^+]=[OH^-]= 2.0 ×10^-3 mol / L . Calculate SOLVED:In a 0.0100 M aqueous solution of methylamine, CH3 NH2, the equilibrium concentrations of the species are [CH3 NH2]=0.0080 mol / L and [CH3 NH3^+]=[OH^-]= 2.0 ×10^-3 mol / L . Calculate](https://cdn.numerade.com/previews/8d40c6fa-0830-4597-a8aa-ec88a39d6189_large.jpg)
SOLVED:In a 0.0100 M aqueous solution of methylamine, CH3 NH2, the equilibrium concentrations of the species are [CH3 NH2]=0.0080 mol / L and [CH3 NH3^+]=[OH^-]= 2.0 ×10^-3 mol / L . Calculate