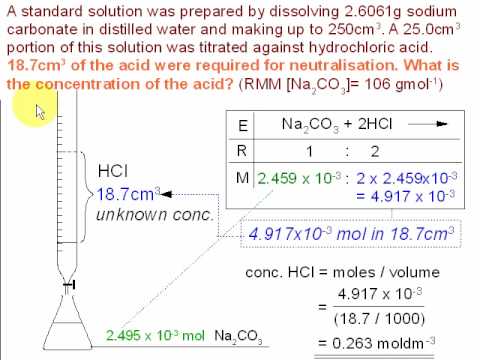
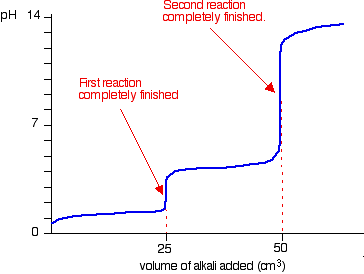
Explain how a pH meter could be used to find the exact volume of acid required to completely react with a sodium carbonate solution? - Quora

chem1-titration sodium carbonate - Name : Nikman Adli b. Nor Hashim (M04D) Title : Standardization of hydrochloric acid using sodium | Course Hero
![SOLVED: Results: TITRATION OF SODIUM CARBONATE WITH HYDROCHLORIC AcID Titration No.L Titration No Titration Noj Initial Final Initial Final Initial Final ACCURATE BURETTE READINGS Final Volume of Sodium Carbonate Cm] Initial Volume SOLVED: Results: TITRATION OF SODIUM CARBONATE WITH HYDROCHLORIC AcID Titration No.L Titration No Titration Noj Initial Final Initial Final Initial Final ACCURATE BURETTE READINGS Final Volume of Sodium Carbonate Cm] Initial Volume](https://cdn.numerade.com/ask_images/5c05cda73576456985cff19309180b31.jpg)
SOLVED: Results: TITRATION OF SODIUM CARBONATE WITH HYDROCHLORIC AcID Titration No.L Titration No Titration Noj Initial Final Initial Final Initial Final ACCURATE BURETTE READINGS Final Volume of Sodium Carbonate Cm] Initial Volume

Sodium Carbonate + Hydrochloric Acid - Na2CO3 + HCl - Molecular Equations & Net Ionic Equations - YouTube

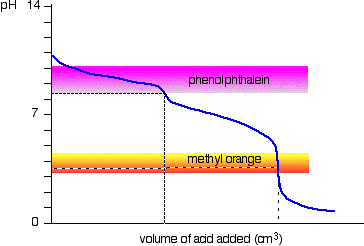
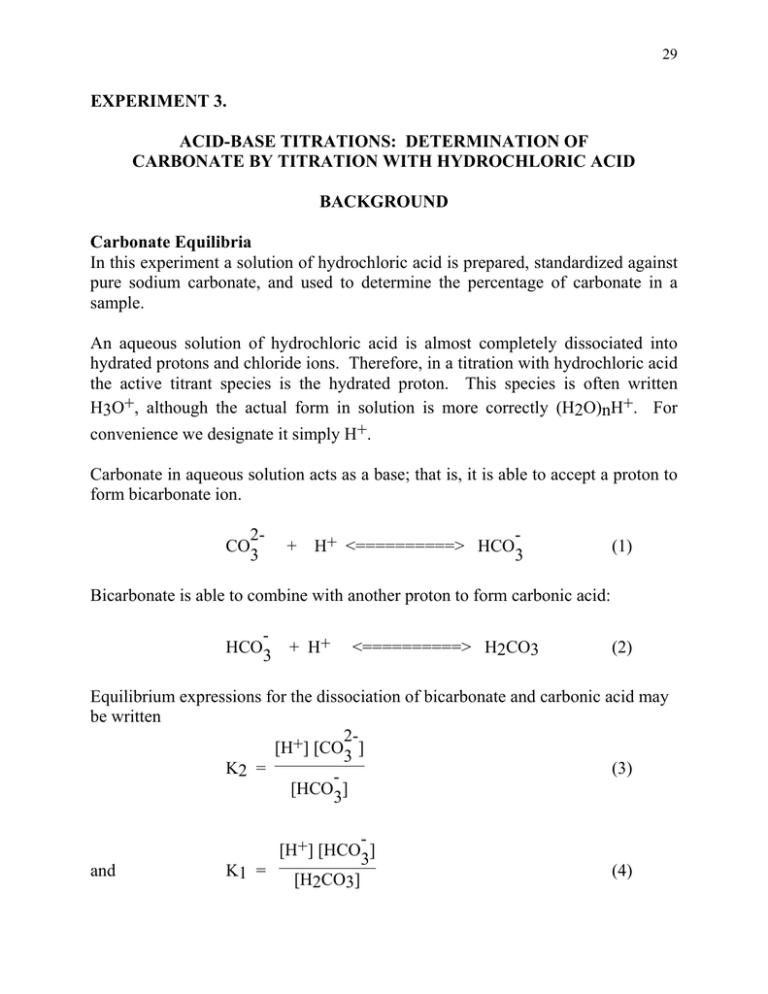
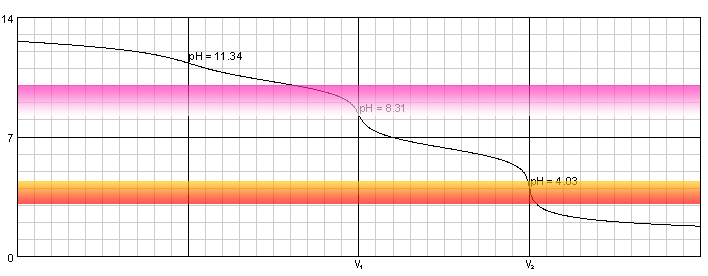
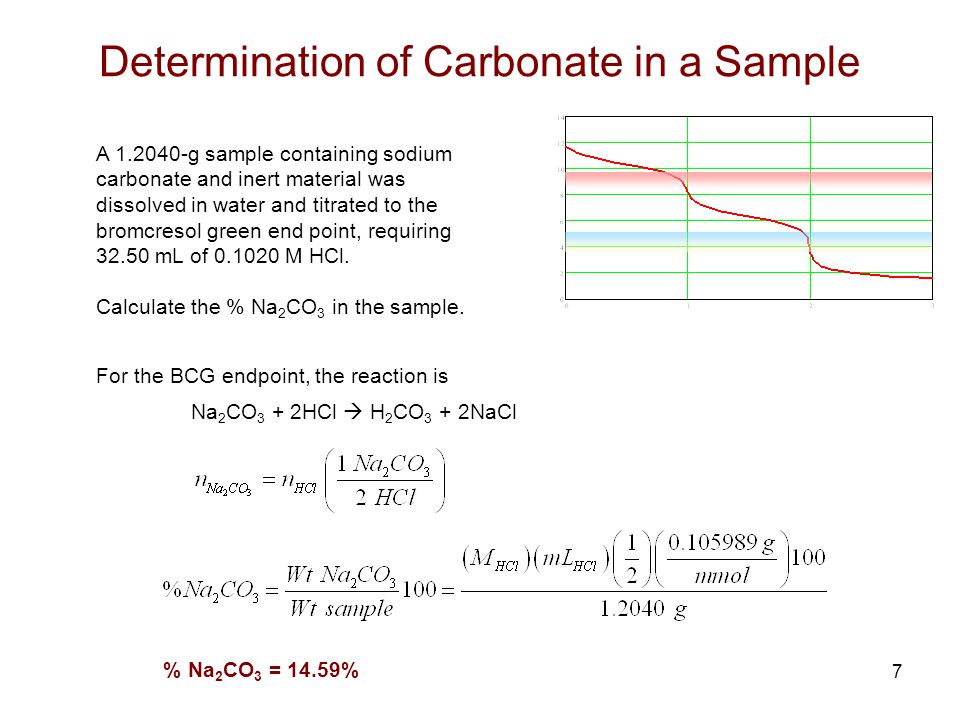
During the titration of sodium carbonate with H Cl, the dissolved carbonate ion will exist in three different forms; CO_3^{-2}, H CO_3^{-1}, and H_2 CO_3. During which part of the titration (initial,
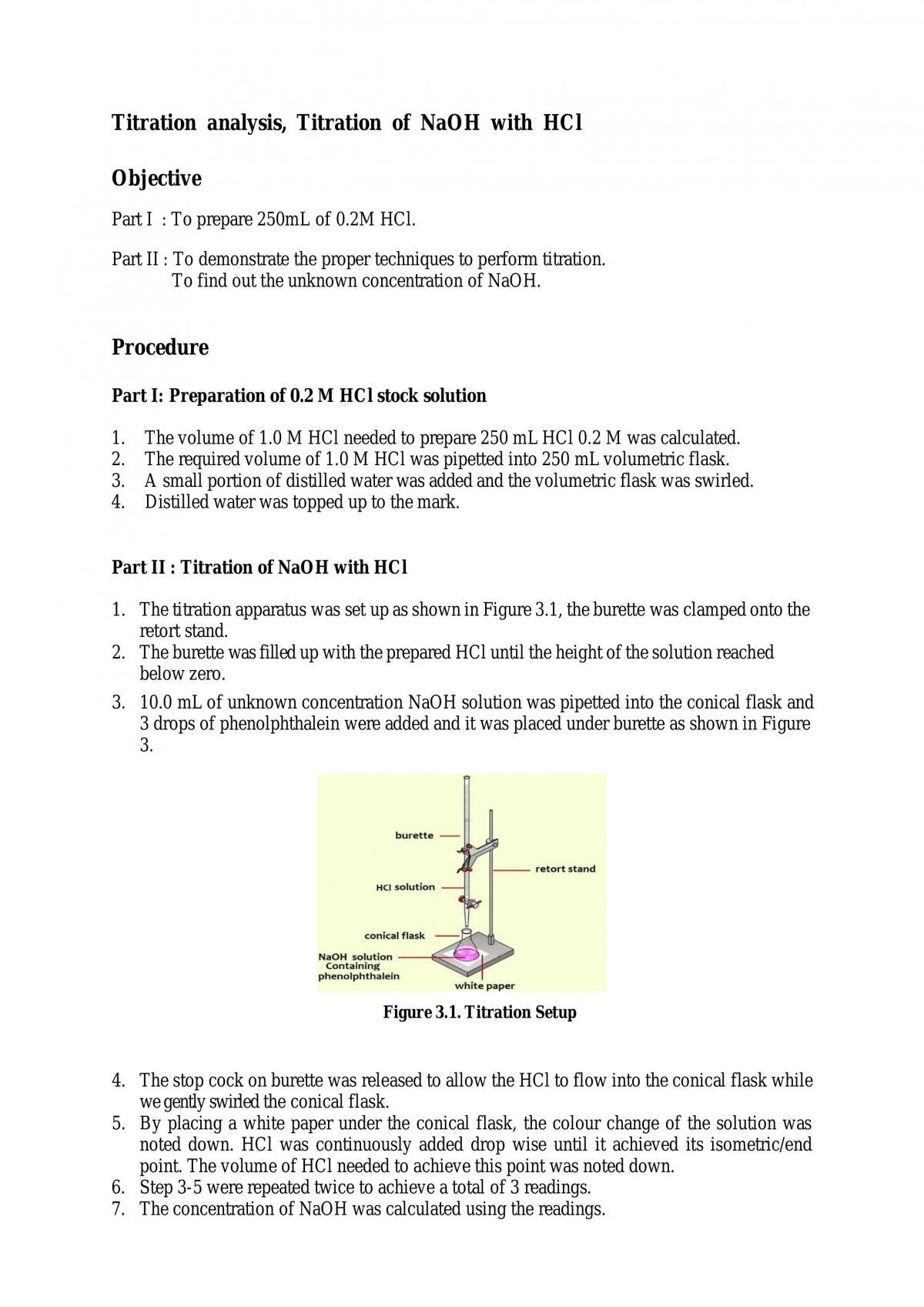
Titration of Sodium Hydroxide with Hydrochloric acid | FSC107 - General Chemistry Laboratory - XMUM | Thinkswap